We find that when water vapor is frozen on a cold surface at T < 30 K, its structure is similar to pressure induced high-density amorphous ice.
Water vapor is bleeded into the transmission electron microscope (a modified Hitachi H-500) until the pressure rises to 2-3 x 10-6 torr. The shutter covering a cooled (15 K) copper grid is opened for 10 seconds. A layer of 0.05 micron thick ice is vapor deposited. Deposition is followed by Selected Area Electron Diffraction. Pressure and gas background are measured with a Quadrupole Mass Spectrometer at the pole piece.
We find that the ice is a smooth layer with no variations in diffraction intensity. The electron diffraction pattern is amorphous, just as with vapor deposits at higher temperature, but the peak of the first diffraction maximum is shifted. With gradual warming (32K) the peak shifts nearer to the central beam, while the second diffraction maximum does not.
Fourrier transform of the diffraction patterns at low and high temperature show that the low-temperature form has O-O distances in between the first and second nearest neighbour of the high-temperature form. The patterns are near-identical with those of pressure induced High Density Amorphous Ice HDA and Low Density Amorphous Ice (LDA). This is the radial distribution function of Bizid et al. (32K)
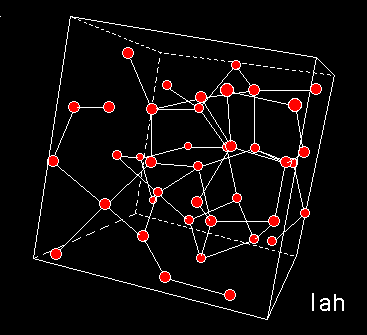
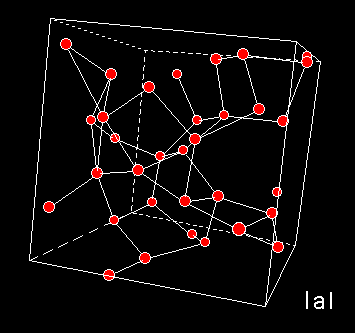
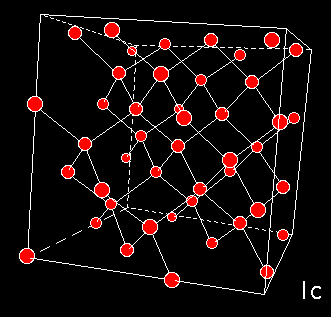
A numerical model of High Density Amorphous ice made by quenching configurations of liquid water, shows that this form of ice lacks the large cavities. Inelastic Neutron Diffraction studies indicate torsion of hydrogen bonding. The network is distorted whereby oxygen atoms have moved into the open cavities of low-density amorphous ice. The pictures to the left show the models of high (top) and low (middle) density amorphous water ice. The bottom figure shows cubic crystalline ice. The red balls are oxygen atoms, the lines are hydrogen bonds.
The range of temperature and deposition rate that leads to the formation of vapor deposited high-density amorphous ice. Crosses indicate the other form, the low-density amorphous ice.
High density amorphous ice is the most abundant ice in the universe, where it is found as a frost on interstellar grains. This ice is the craddle where small inorganic molecules combine into large organic molecules that may be at the origin of life.
Last modified: July 7, 1997
P. Jenniskens
D.F. Blake