|
Leonid MAC |
| home |
| View the shower |
| Mission Brief |
| Science Update |
| Media Brief |
| links |
LEONID DAILY NEWS: November 13, 2000
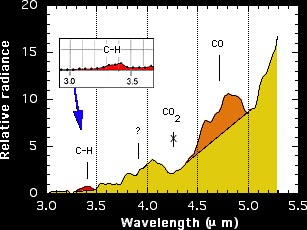
Figure right: The measured mid-Infrared spectrum. Relative radiance
is plotted versus wavelength between 3 and 5.5 micron.
ORGANIC FINGERPRINT OF COMET DUST FOUND IN METEOR TRAIN EMISSION
The Leonid Multi-Instrument Aircraft Campaing is NASA's first astrobiology
mission. Its mission is to study the link between the abundant organic matter found in comets
and interstellar matter in space, and the terrestrial environment that generated
life on our planet.
Potential new ways of bringing organic matter to Earth
by ways of meteor ablation were reported on earlier. Now,
in today's issue of Earth, Moon and Planets
Ray Russell,
George Rossano,
Mark Chatelain, David Lynch,
Ted Tessensohn,
Eric Abendroth and Daryl Kim of
The Aerospace
Corporation and Peter Jenniskens of the
SETI Institute,
at NASA Ames Research Center, report on a different
manner how organic matter may have survived the plunge into Earth's atmosphere.
Pointing their infrared telescope at the persistent train in
the path of the Y2K
fireball, they discovered the fingerprint of complex organic matter: the C-H
stretch vibration band at 3.4 micron. In addition a continuum emission was observed,
possible from warm dust, and an emisison band that is caused by warm CO molecules in
the path of the meteor.
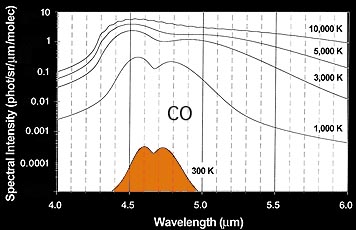
Figure: The model spectrum for warm CO emission.
The organic C-H band is not well resolved, but it has much the same shape as the
organic matter found in the dust of comets. This suggests that the organic matter
survived more or less in-tact.
Other instruments deployed during the mission
showed that the fireball left
meteoric debris behind. Organic matter in comet dust is intimately mixed with
mineral grains. Hence, the observations suggest that organic matter can survive in
the meteoric debris after deposition in the atmosphere.
It is possible, however, that trace air compounds such as methane cause the C-H stretch
vibration band. The reason why this feature is a fingerprint of complex organic
matter, is because all molecules containing C-H2 or C-H3 tend to
absorp and emit light at this point in the spectrum. The methane molecule does so as well.
Although the band shape is expected to be different, further modelling and analysis of
more of the data is required to exclude any contribution from such source. Finally, rather
than trace air compounds, the methane might be formed if the
meteor material could have been broken down into atomic species and then formed into the
observed molecules. All these alternatives need further study.
Cabon monoxide (CO) was detected with certainty. The CO molecule could be a trace air compound,
but can also be created from atmospheric carbon dioxide CO2 in the path
of the meteor. The model calculations left show that the CO molecules have a
temperature of about room temperature (300K) at the time of the measurement several minutes
after the fireball, some 50 degrees
above the ambient temperature at that altitude. That temperature is consistent with
earlier measurements of the sodium atom
temperature by LIDAR and the
temperature decay measured from the meteoric
metal atom emissions (Full paper - PDF)
Nov. 13 - Organic fingerprint Nov. 12 - Train airglow chemistry Nov. 11 - Hard bits and persisting glows Nov. 10 - Meteoroid debris detected Nov. 09 - New meteor picture Nov. 08 - Spin city Nov. 07 - Meteors affect atmospheric chemistry Nov. 06 - Listen to this! Nov. 04 - Fear of heights? Nov. 03 - The pale (infra-red) dot Nov. 02 - Twin showers Nov. 01 - Leonids approaching Earth Oct. 31 - Prospects for Moon Impact Studies Oct. 30 - Comet dust crumbled less fine Today's news
| ||