|
Leonid MAC |
| home |
| View the shower |
| Mission Brief |
| Science Update |
| Media Brief |
| links |
LEONID DAILY NEWS: November 12, 2000
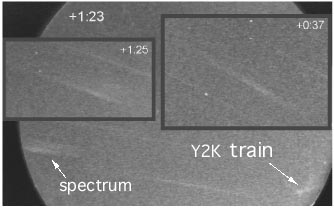
Figure right: A remarkable video record of the Y2K persistent train
emission.
TRAIN AIRGLOW CHEMISTRY
Persistent trains were first described during the 1866 Leonid storm. Once bright
fireball would have faded, a persistent glow was seen that sometimes persisted for several
tens of minutes. Ever since, researchers have wondered why the glows persist so long
and what is causing the visible emission.
Now, that secret has been partially unlocked. In an upcoming issue of Earth, Moon
and Planets,
Peter Jenniskens and Matt Lacey
at NASA Ames Research Center and
Beverley Allan, Daniel Self and
John Plane of the University of East Anglia, UK,
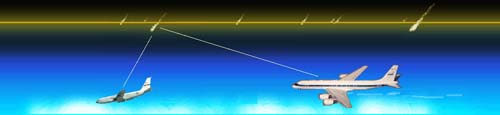
report on the first calibrated optical spectroscopy of persistent trains. The
measurements were taken onboard the ARIA aircraft participating in the
Leonid Multi-Instrument Aircraft Campaign and show that the visible emission of
persistent trains is the result of chemistry that also causes the natural airglow.
Until now, only slit-less spectra of trains were obtained.
Jiri Borovicka measured
the rare train spectrum shown below left
of the "Y2K" persistent train.
The spectrum of the train is the fuzzy band to the left of the image of the train, seen
in the picture above, and
is created by using an objective grating in front of the camera.
Features in the spectra smear out when the train diffuses and
the wavelength calibration is always somewhat uncertain
in this type of measurements. Hence, the origin of the band emission remained in doubt.
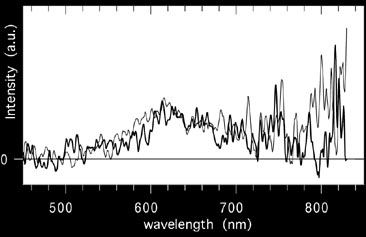
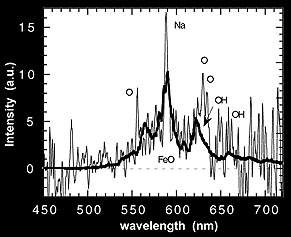
This figure shows a trace of the slit-less spectrum.
Figure
shows the result using the slit spectrograph. Both spectra show a broad emission band.
The slit-spectroscopy, however, points a telescope at the glow, collects the light
into a fibre and leads it into a spectrograph. Thus, a spectrum is obtained for which
the wavelength scale can be calibrated. Comparisson with laboratory data identified
the band as caused by iron-oxide (Full paper - PDF).
The spectroscopic observations confirm that most intense emission arrises from the
sodium (Na) D-line and from the molecular emission band of iron-oxide (FeO). These
emissions occur almost certainly through the Chapman airglow mechanism, where oxygen
atoms and ozone molecules recombine to oxygen molecules in a process that is catalysed
by the meteoric metals sodium (Na) and iron (Fe):
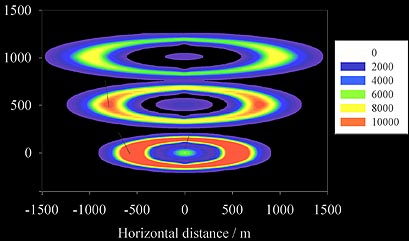
The new train model.
Subsequent modeling by
John Plane of the University
of East Anglia
showed that the tubular structure can be explained
if the oxygen atoms are created by the fireball, while the ozone is that in the
ambient atmosphere. Diffusion processes bring the catalytic metal atoms out to the
edge where ambient ozone diffuses into the train environment, and are responsible
for the gradual expansion of the trail shown by the model cross section at three different
times in the figure above (Full paper - PDF).
Many fundamental questions remain. The model does not yet explain why the trains are so dark in
the center and why they expand linearly in time. It is clear that persistent trains can
help understand the physical processes that lead to the natural airglow in the upper atmosphere
by setting constraints on diffusion timescales and
relevant chemistry mechanisms.
Nov. 12 - Train airglow chemistry Nov. 11 - Hard bits and persisting glows Nov. 10 - Meteoroid debris detected Nov. 09 - New meteor picture Nov. 08 - Spin city Nov. 07 - Meteors affect atmospheric chemistry Nov. 06 - Listen to this! Nov. 04 - Fear of heights? Nov. 03 - The pale (infra-red) dot Nov. 02 - Twin showers Nov. 01 - Leonids approaching Earth Oct. 31 - Prospects for Moon Impact Studies Oct. 30 - Comet dust crumbled less fine Today's news
| ||

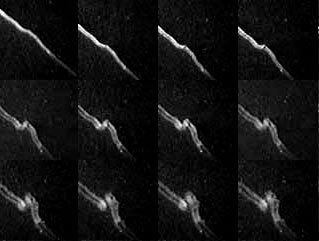 Images of the Chippenham train show two parallel structures.
It is now also suspected where the ozone and oxygen come from.
Many trains turn out to have a tubular structure, with two bands of intense emission
where the line of sight crosses the edge of the tube.
Images of the Chippenham train show two parallel structures.
It is now also suspected where the ozone and oxygen come from.
Many trains turn out to have a tubular structure, with two bands of intense emission
where the line of sight crosses the edge of the tube.